Helium Nucleus Given Off By Radioactive Substances
Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed. It is a silvery-grey metal in the actinide series of the periodic tableA uranium atom has 92 protons and 92 electrons of which 6 are valence electrons.

Electromagnetic Radiation Gamma Rays Britannica
The nucleus is made of one or more protons and a number of neutronsOnly the most common variety of hydrogen has no neutrons.
. The official video for Never Gonna Give You Up by Rick AstleyTaken from the album Whenever You Need Somebody deluxe 2CD and digital deluxe out 6th May. The seventh period is a blank period. This is approximately the sum of the number of protons and neutrons in the nucleus.
ASCII characters only characters found on a standard US keyboard. Radioactive waste is a type of hazardous waste that contains radioactive materialRadioactive waste is a result of many activities including nuclear medicine nuclear research nuclear power generation rare-earth mining and nuclear weapons reprocessing. Every solid liquid gas and plasma is composed of neutral or ionized atoms.
Must contain at least 4 different symbols. Formally a string is a finite ordered sequence of characters such as letters digits or spaces. This lets us find the most appropriate writer for any type of assignment.
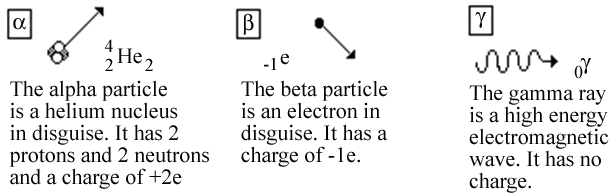
The empty string is the special case where the sequence has length zero so there are no symbols in the string. The emission of energy from radioactivity is always accompanied by alpha beta and gamma particles. 6 to 30 characters long.
Uranium is a chemical element with the symbol U and atomic number 92. 7 November 1878 27 October 1968 was an Austrian-Swedish physicist who was one of those responsible for the discovery of the element protactinium and nuclear fissionWhile working at the Kaiser Wilhelm Institute on radioactivity she discovered the radioactive isotope protactinium-231 in. Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element.
A type of radiation therapy used to treat eye tumors. With a focus on Bohrs work the developments explored in this module were based on the advancements of many scientists over time and laid the groundwork for. For example beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino.
The terms isotope and nuclide are often used synonymously but they refer to. Atomic mass unit See dalton. The sixth period in the periodic table is regarded as the longest period.
How much more of the drug did you receive. Which scientist determined the charge of the. The 20th century brought a major shift in our understanding of the atom from the planetary model that Ernest Rutherford proposed to Niels Bohrs application of quantum theory and waves to the behavior of electrons.
The universe is a vast soup of interacting particles and energy. Its density is about 70 higher than that of lead and slightly lower than. The periodic table also known as the periodic table of the chemical elements is a rows and columns arrangement of the chemical elementsIt is widely used in chemistry physics and other sciences and is generally seen as an icon of chemistry.
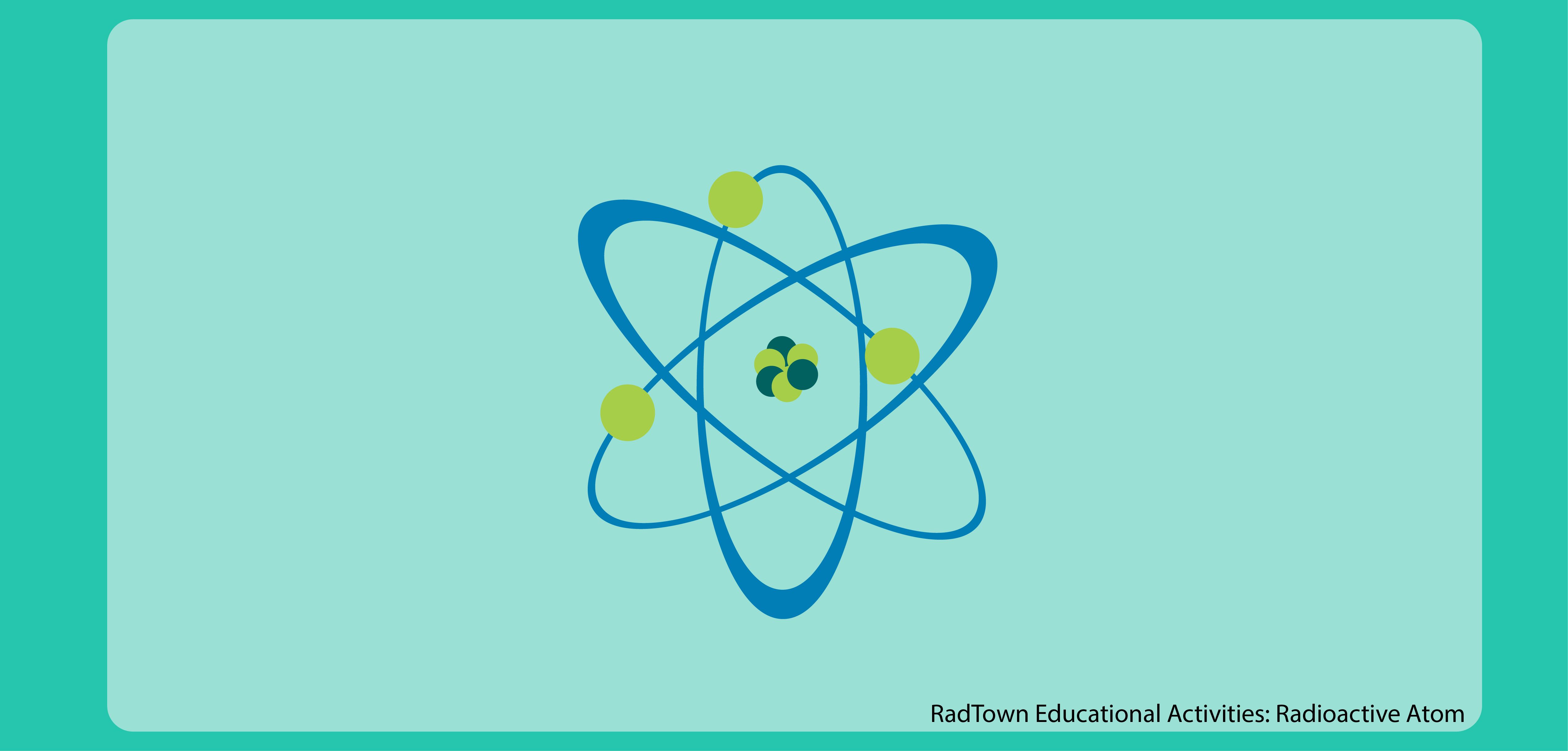
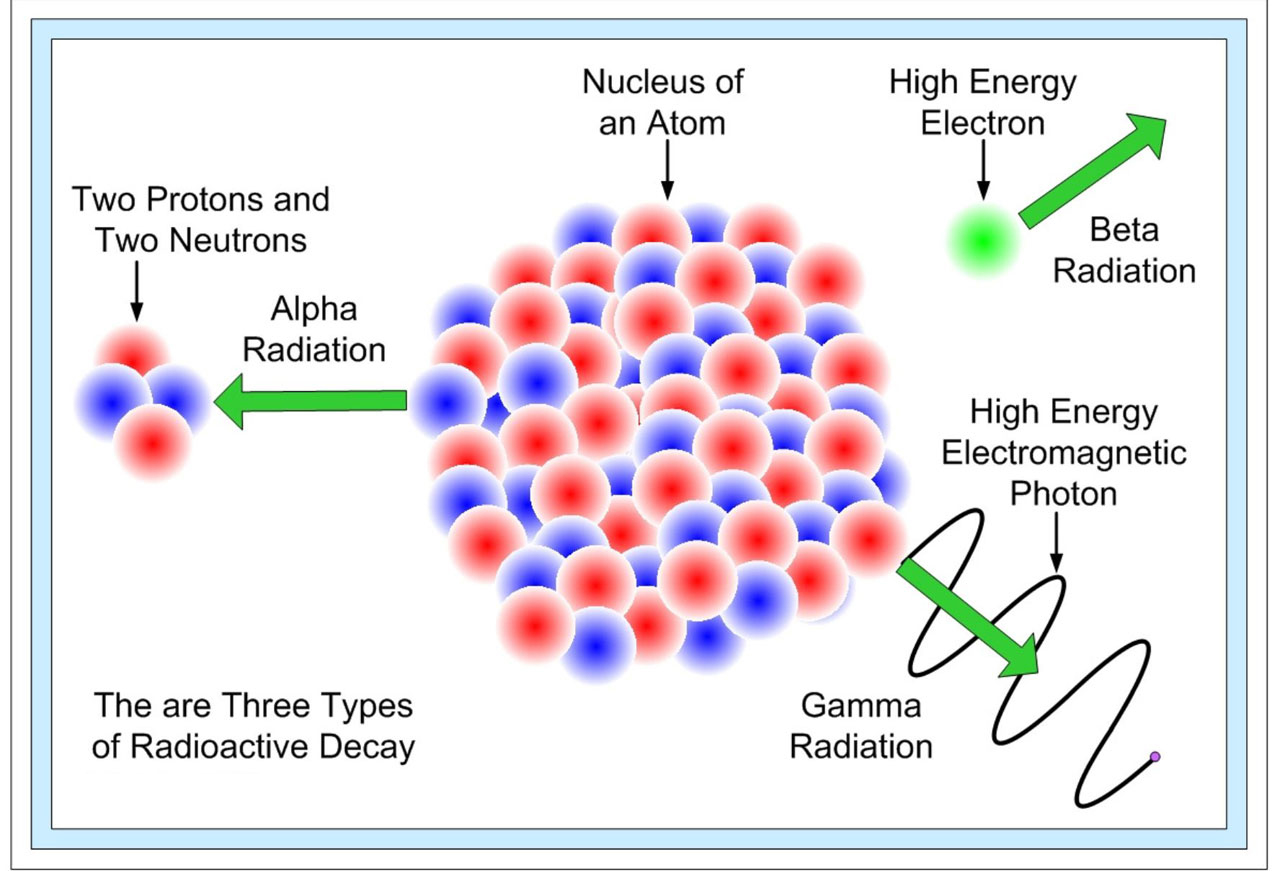
Radioactive decay also known as nuclear decay radioactivity radioactive disintegration or nuclear disintegration is the process by which an unstable atomic nucleus loses energy by radiationA material containing unstable nuclei is considered radioactiveThree of the most common types of decay are alpha decay α-decay beta decay β-decay and gamma decay. Hydrogen is the chemical element with the symbol H and atomic number 1. The mass of an atom relative to that of carbon-12.
The total energy of a system can be subdivided and classified into potential energy kinetic energy or combinations of the two in various ways. An atomic nucleus is formed by a number of protons Z the atomic number and a number of neutrons N the neutron number bound together by the nuclear forceThe atomic number determines the chemical properties of the atom and the neutron number determines the isotope or nuclide. Our chemistry learning modules introduce you to the world of chemistry exploring current research and scientific findings on concepts like the structure and function of.
At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2It is colorless odorless tasteless non-toxic and highly combustibleHydrogen is the most abundant chemical substance in the universe constituting roughly 75 of all normal matter. The ways in which those interactions take place as well as the structure and composition of matter is the main focus of the field of chemistry. Every atom is composed of a nucleus and one or more electrons bound to the nucleus.
In nuclear physics beta decay β-decay is a type of radioactive decay in which a beta particle fast energetic electron or positron is emitted from an atomic nucleus transforming the original nuclide to an isobar of that nuclide. Isotopes Atoms of the same element with different numbers of neutrons. The electrons mass is approximately 11836 that of the proton.
The plaque is sewn onto the outside wall of the eye. Uranium has the highest atomic weight of the primordially occurring elements. The first period which only has two elementshydrogen and heliumis the shortest.
The storage and disposal of radioactive waste is regulated by government agencies in order to protect human health and. You were given a dose of 500 mg rather than 500 μg of a drug. Quantum mechanical properties of the.
Or conversely a proton. Electrons belong to the first generation of the lepton particle family and are generally thought to be elementary particles because they have no known components or substructure. The number of protons found in the nucleus of an atom of a given chemical elementIt is identical to the charge number of the nucleus and is used in the periodic table to.
Elise Meitner ˈ l iː z ə ˈ m aɪ t n ər LEE-zə MYTE-nər German. A chemical element is a species of atoms that have a given number of protons in their nuclei including the pure substance consisting only of that species. All of your shots are off but they all go to the same place.
The rate of decay of radioactive substances is dependent on the number of atoms that are present at the time. Unlike chemical compounds chemical elements cannot be broken down into simpler substances by any chemical reactionThe number of protons in the nucleus is the defining property of an element and is referred to as. Positively charged particles of radiation emitted from the decay of radioactive substances are known as.
Atoms are extremely small typically around 100 picometers across. Kinetic energy is determined by the movement of an object or the composite motion of the components of an object and potential energy reflects the potential of an object to have motion and generally is a function of the. The electron e or β is a subatomic particle with a negative one elementary electric charge.
The plaque is removed at the end of treatment which usually lasts for several days. It is a graphic formulation of the periodic law which states that the properties of the chemical elements exhibit an approximate periodic. The physical and chemical properties of the daughter nucleus are different from the mother nucleus.
Where more than one isotope exists the value given is the abundance weighted average. Our global writing staff includes experienced ENL ESL academic writers in a variety of disciplines. It contains substances ranging from Radon to Cesium.
A transmutation can be achieved either by nuclear reactions in which an outside particle reacts with a nucleus or by radioactive decay. The seeds give off radiation which kills the cancer. Radioactive seeds are placed on one side of a thin piece of metal usually gold called a plaque.
Actinides and Lanthanides are included at the bottom of the periodic table. Atomic mass The mass of an atom typically expressed in daltons and nearly equivalent to the mass number multiplied by one dalton. Hydrogen is the lightest element.
Radioactivity
Radioactivity Law Of Radioactive Decay Decay Rate Half Mean Life Q A

Radioactivity And The Decay Of Nuclei
Radioactive Decay

Lesson Video Radioactivity Nagwa

What Are Radioactive Substances Examples Uses Video Lesson Transcript Study Com

Solved Which Is Not Emitted By Radioactive Substance A A Rays B B Rays C Positron D Proton

Alpha Decay

Alpha Decay
When Radioactive Material Decays It Emits Alpha Particles Why Does The Nuclei Not Split In Other Ways Why Is It Always A Helium Nuclei Quora
Alpha Decay School Of Physics
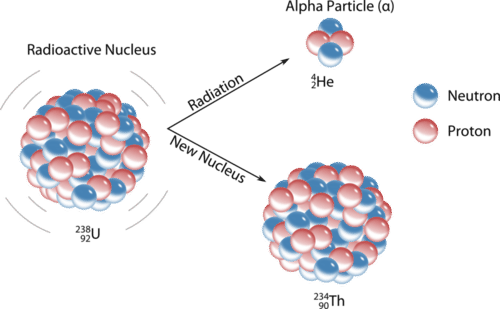
10 1 Nuclear Radiation Chemistry Libretexts
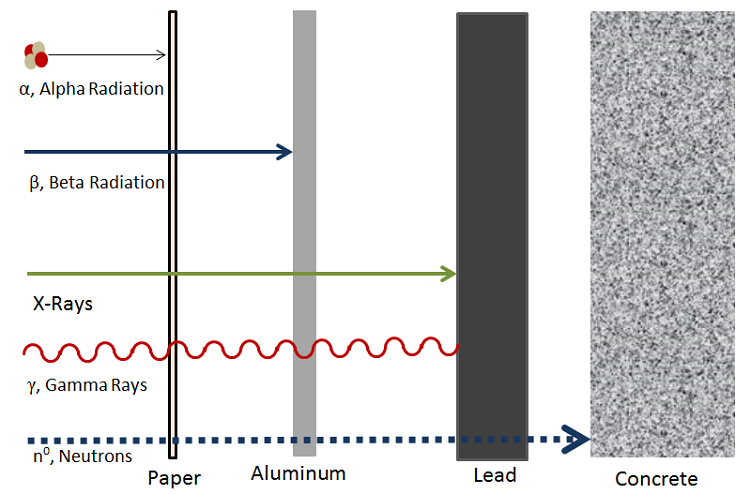
Alpha Decay Energy Education
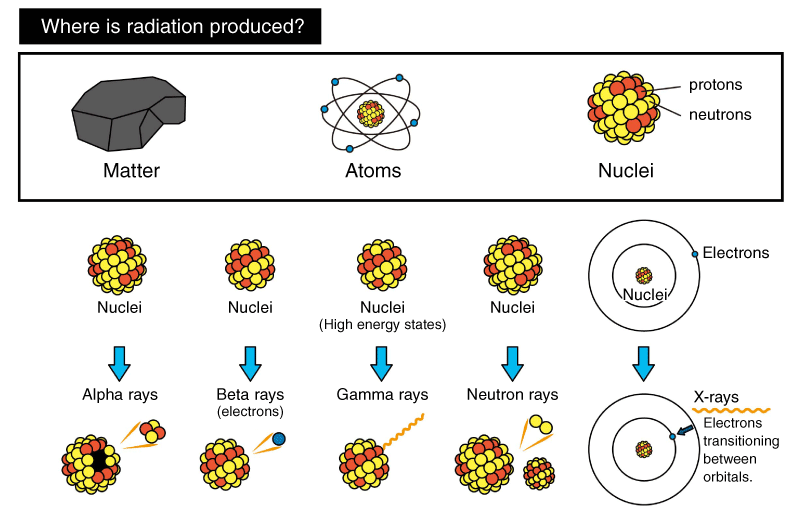
Radioactivity And Radiation Tech Matsusada Precision

Notes Radioactivity

What Is Alpha Decay With Pictures
Gcse Nuclear Radiation Types Of Radioactivity